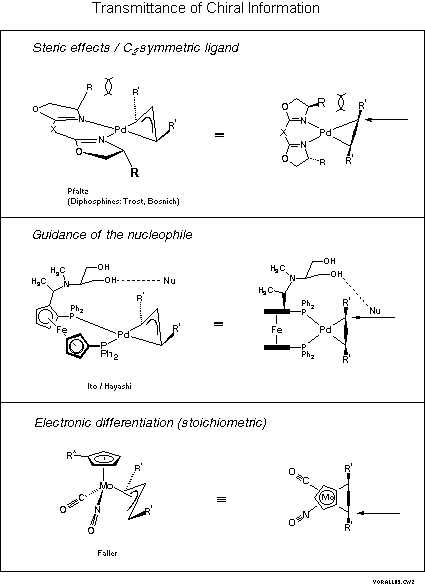
Determination of Absolute Configuration of (π‐Allyl)Palladium Complexes by NMR Spectroscopy and Stereoselective Complexation - Gogoll - 2001 - Chemistry – A European Journal - Wiley Online Library
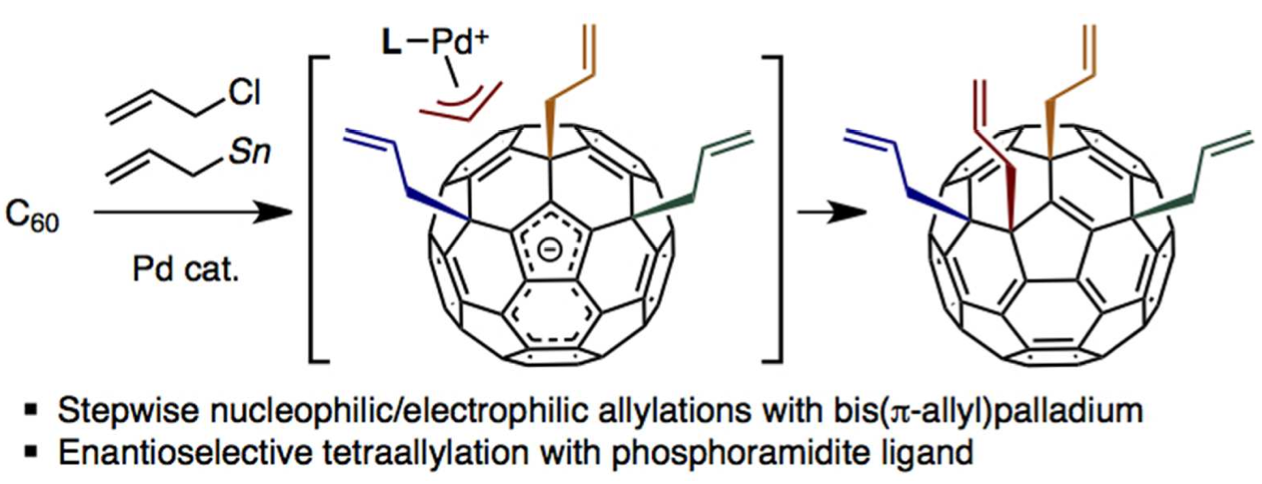
Palladium-catalyzed tetraallylation of C60 with allyl chloride and allylstannane: Mechanism, regioselectivity, and enantioselectivity | Itami Organic Chemistry Laboratory, Nagoya University

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society

Palladium-Catalyzed Asymmetric Allylic Alkylation/α-Iminol Rearrangement: A Facile Access to 2-Spirocyclic-Indoline Derivatives | CCS Chem

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society
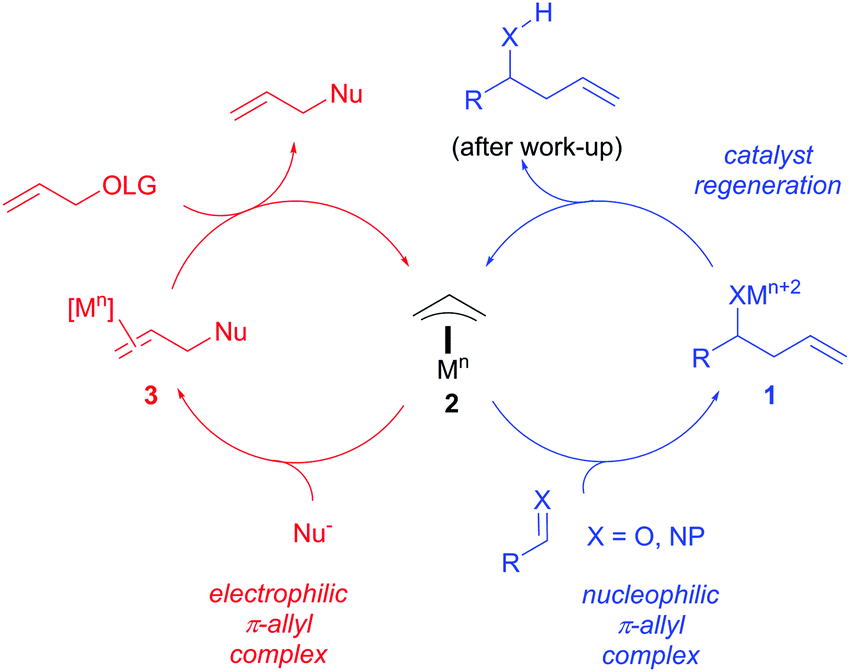
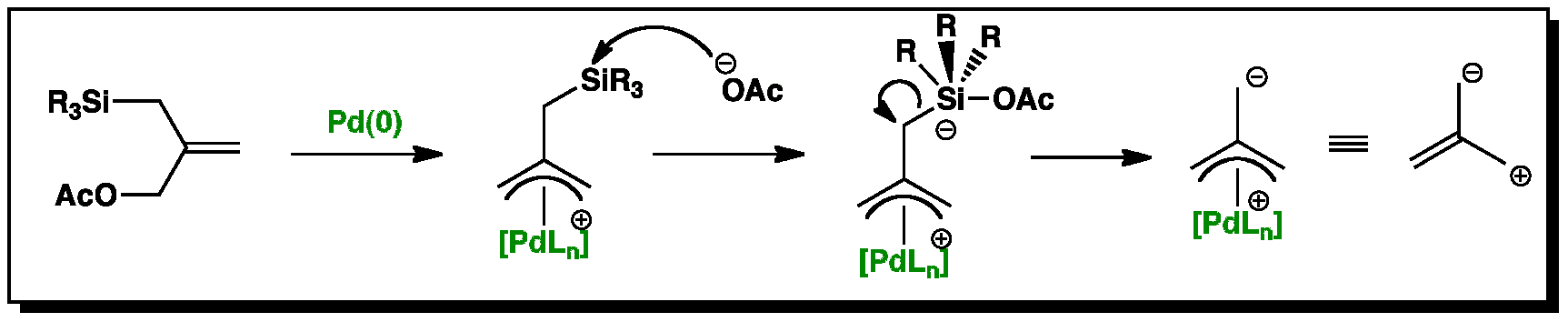
Catalytic nucleophilic 'umpoled' π-allyl reagents - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C7CS00449D
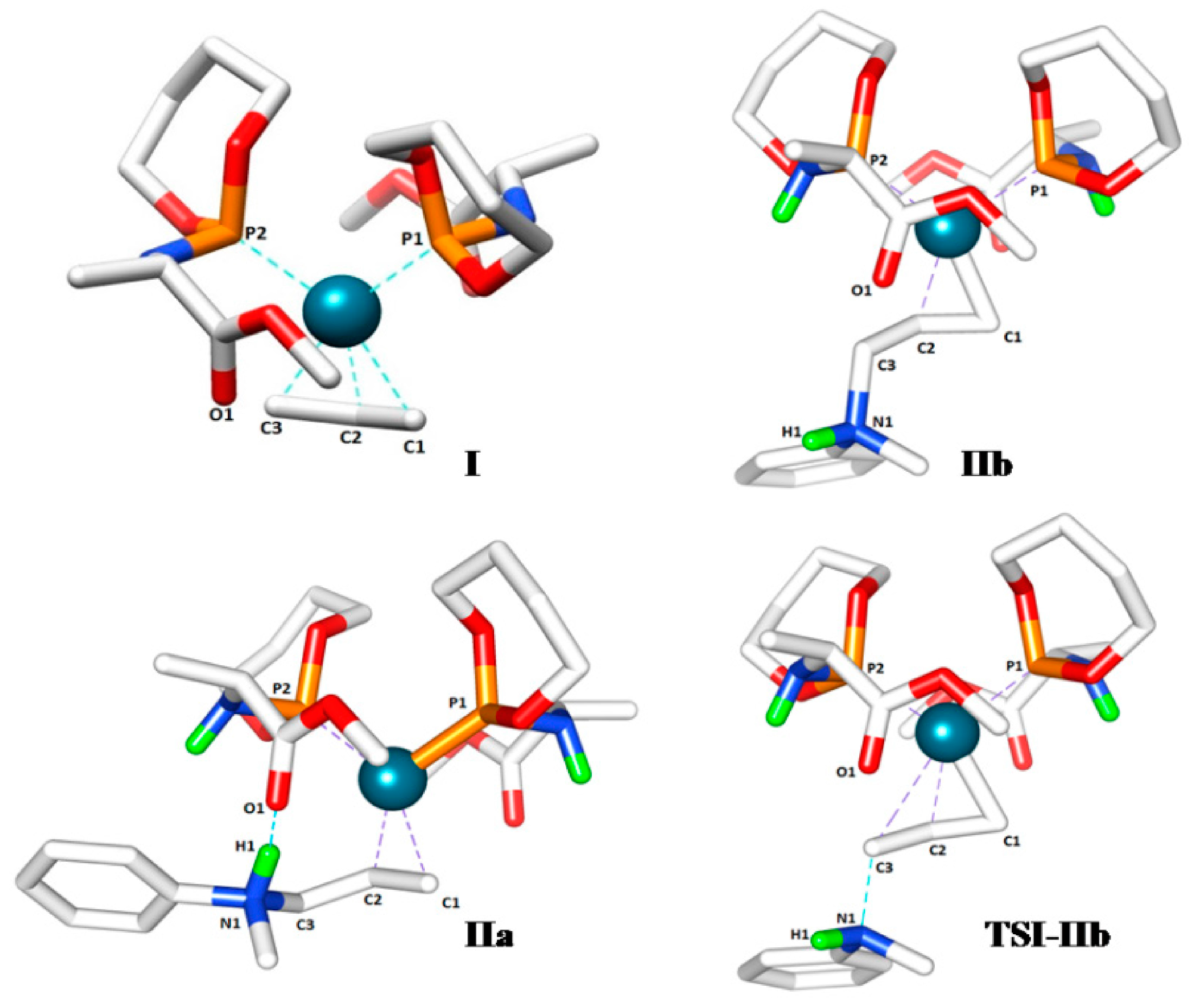
Catalysts | Free Full-Text | A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions
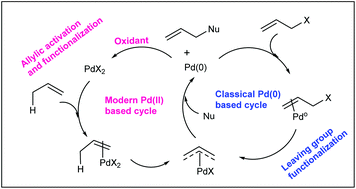
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)

Palladium-Catalyzed Asymmetric Allylic Alkylation/α-Iminol Rearrangement: A Facile Access to 2-Spirocyclic-Indoline Derivatives | CCS Chem

The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst

Palladium‐Catalyzed Electrophilic Allylation Reactions via Bis(allyl) palladium Complexes and Related Intermediates - Szabó - 2004 - Chemistry – A European Journal - Wiley Online Library

Stereochemistry of the palladium-catalyzed allylic substitution: the syn-anti dichotomy in the formation of (π-allyl)palladium complexes and their equilibration - ScienceDirect

Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect