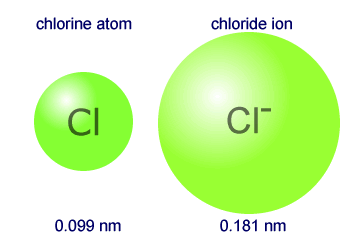
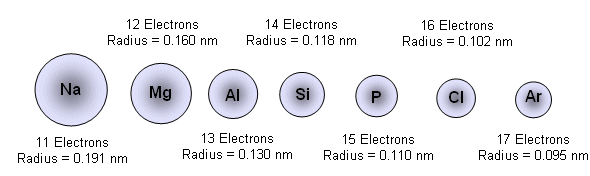
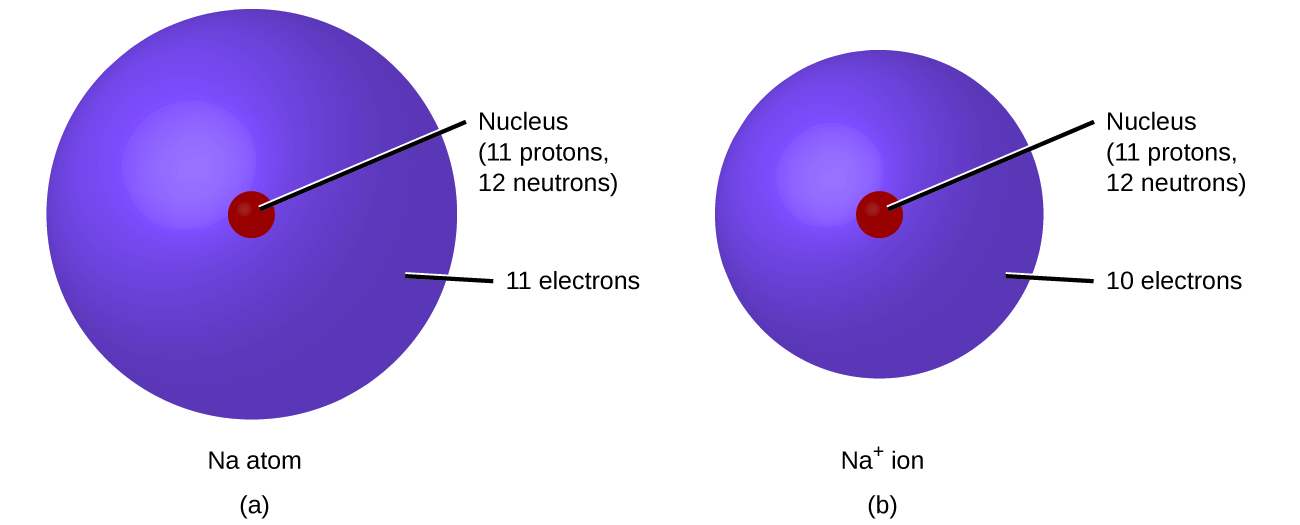
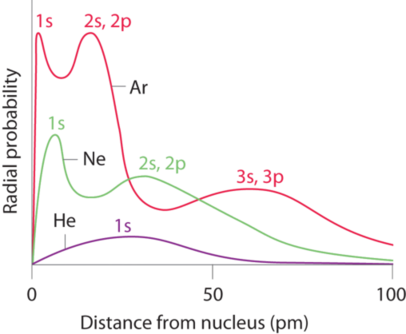
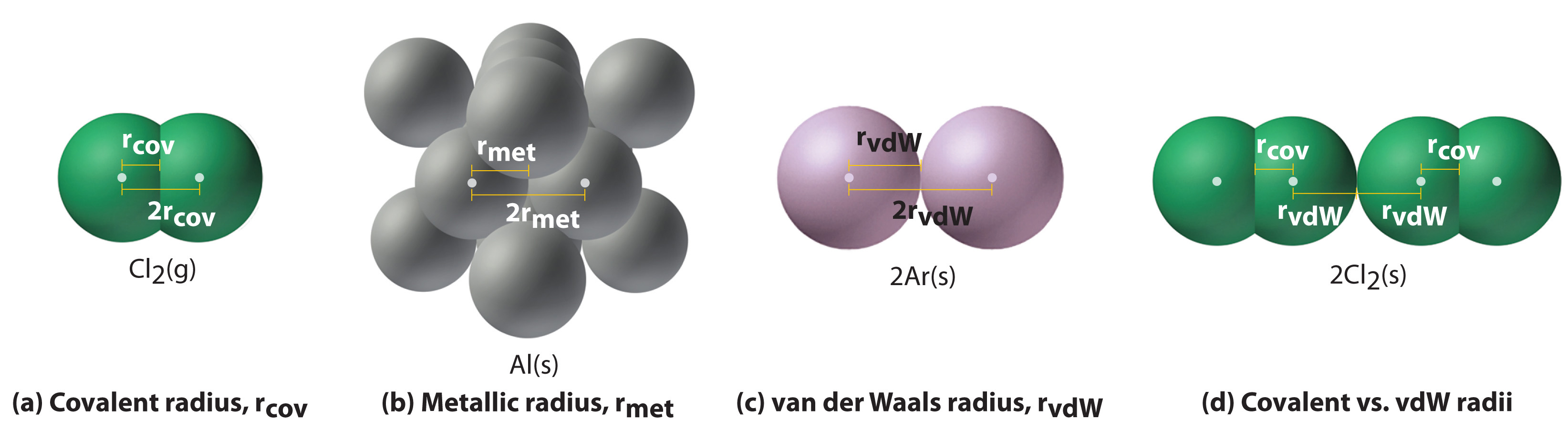
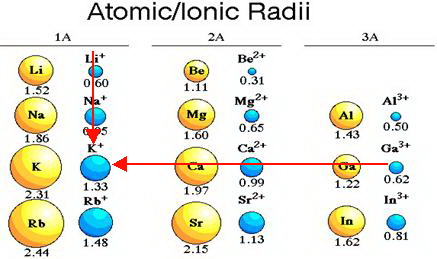
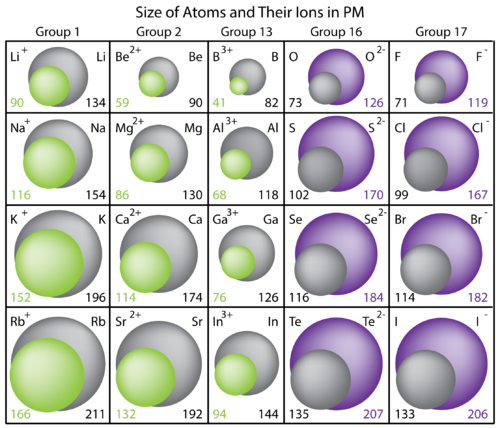
Why the size of Sodium atom is greater then sodium ion?size of Flourine atom is smaller then its ion - YouTube

From Lithium‐Ion to Sodium‐Ion Batteries: Advantages, Challenges, and Surprises - Nayak - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Towards K‐Ion and Na‐Ion Batteries as “Beyond Li‐Ion” - Kubota - 2018 - The Chemical Record - Wiley Online Library

High Stability and Long Cycle Life of Rechargeable Sodium-Ion Battery Using Manganese Oxide Cathode: A Combined Density Functional Theory (DFT) and Experimental Study | ACS Applied Materials & Interfaces
![Frontiers | Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1–6)]: An ab initio Study on Structures and Non-covalent Interaction Frontiers | Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1–6)]: An ab initio Study on Structures and Non-covalent Interaction](https://www.frontiersin.org/files/Articles/468925/fchem-07-00624-HTML/image_m/fchem-07-00624-g001.jpg)
Frontiers | Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1–6)]: An ab initio Study on Structures and Non-covalent Interaction

Structural engineering of electrode materials to boost high-performance sodium-ion batteries - ScienceDirect

The Role of a Sodium Ion Binding Site in the Allosteric Modulation of the A2A Adenosine G Protein-Coupled Receptor - ScienceDirect
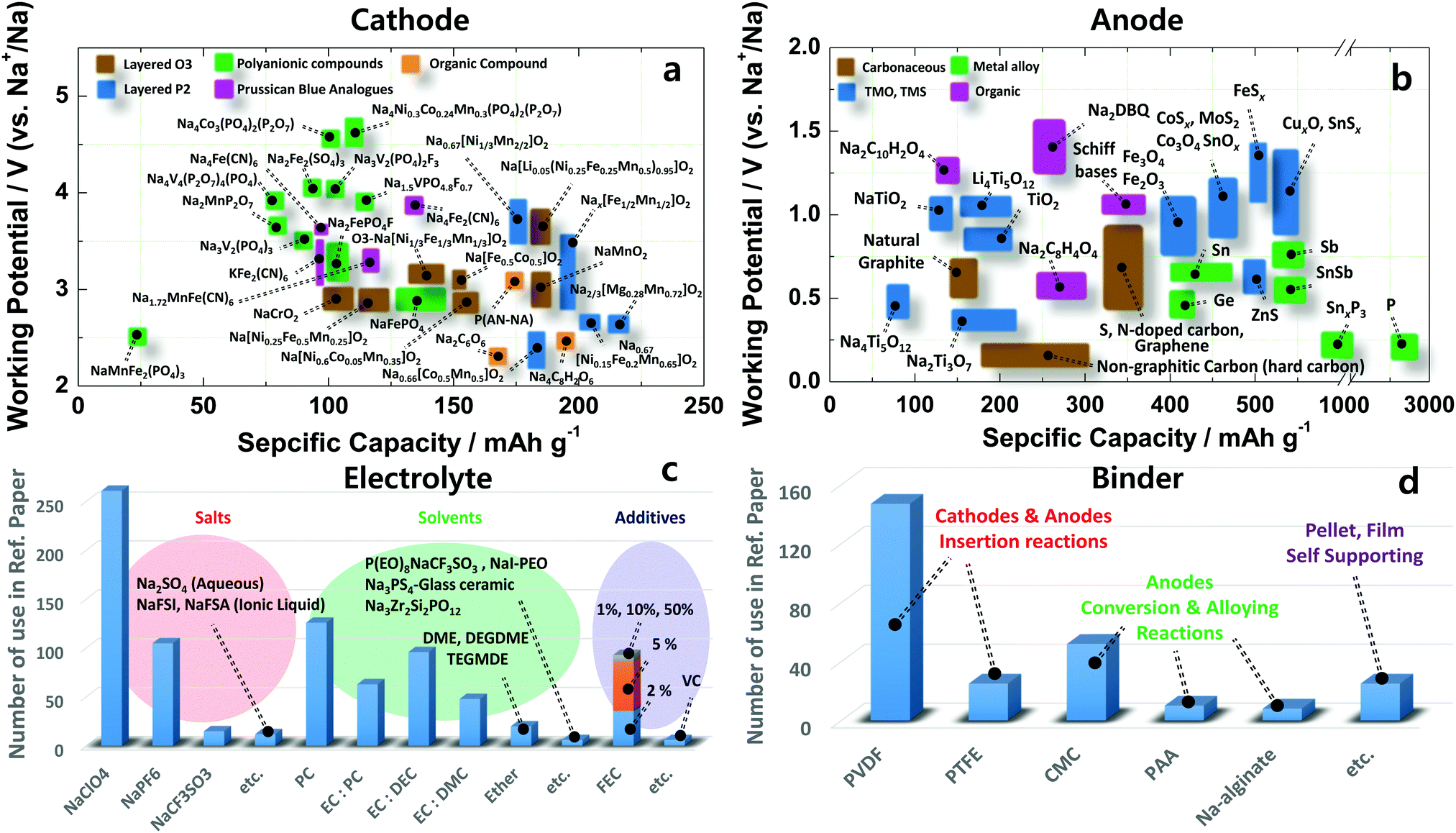
Sodium-ion batteries: present and future - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C6CS00776G